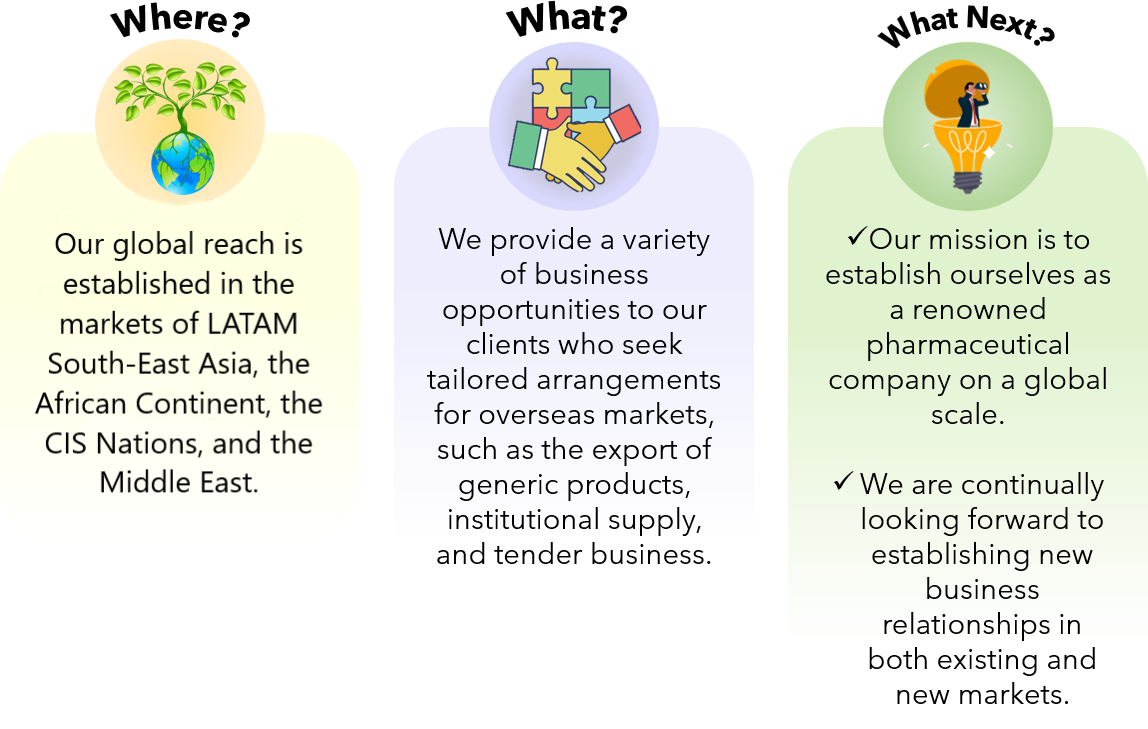
With additional products registered a strong belief in quality, we aim to reach new markets and anticipate filing more dossiers in the upcoming months. We currently have about 100 marketing authorizations in various parts of the world with many more coming in the near future.
The international marketing is supported by the Regulatory, Marketing and Business Development team whose primary objective is to offer timely and best-in-class support to the foreign distributors and medical community. A savvy blend of vastly experienced professionals, young and highly skilled technocrats are commited to to collaborate closely with company’s distributors and abroad clients in order to build an efficient marketing network. The business is prepared to provide all necessary regulatory, technical, and promotional assistance.
If you are looking to launch a new product line or broaden your existing portfolios in international markets, Medople is the perfect place to be with!